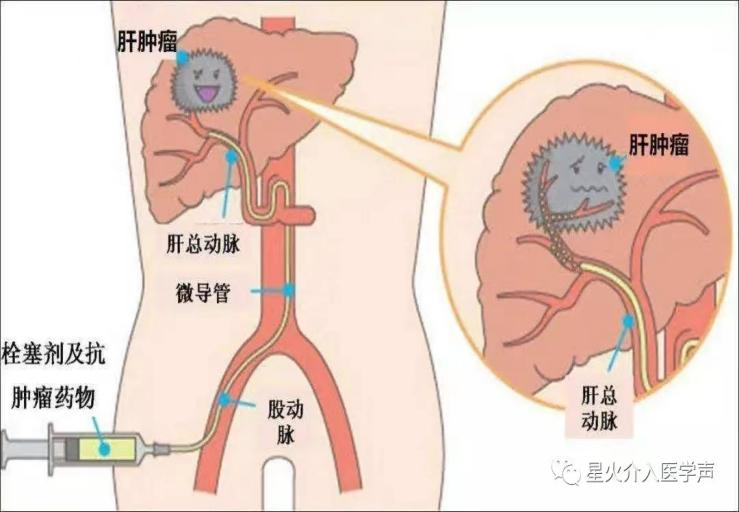
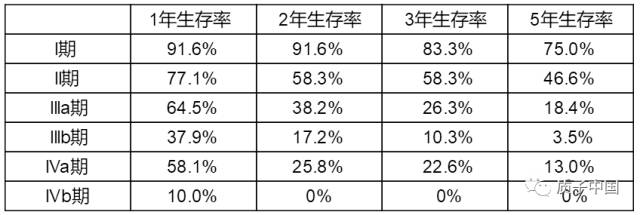
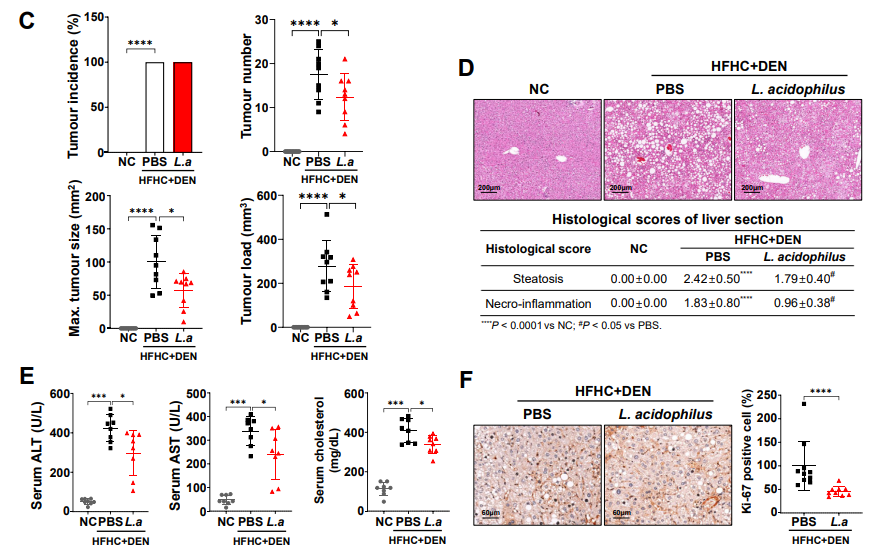
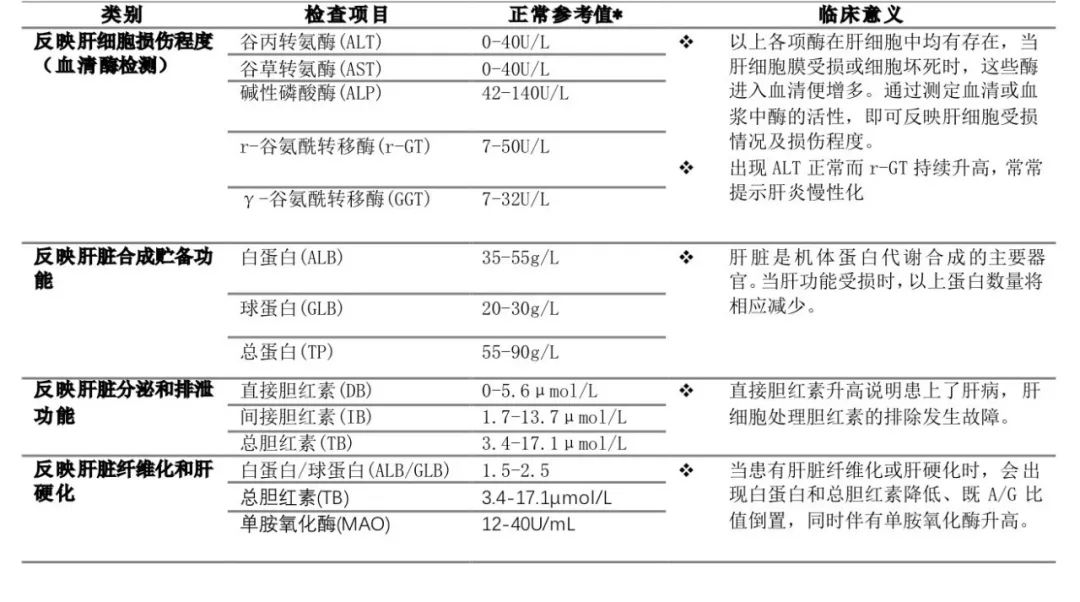
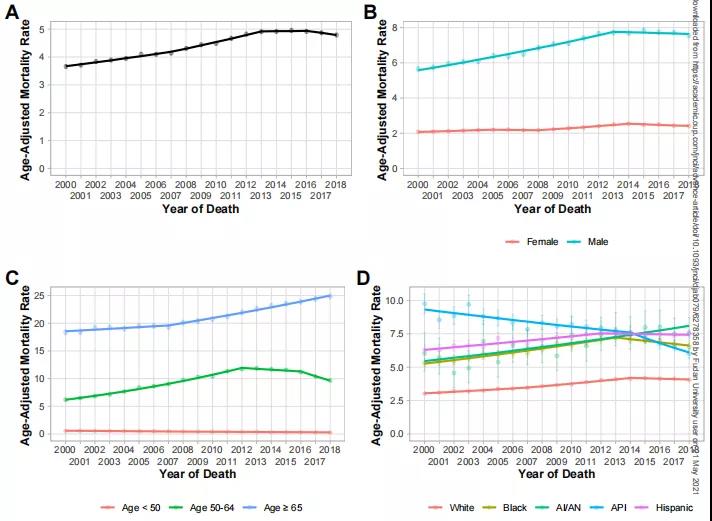
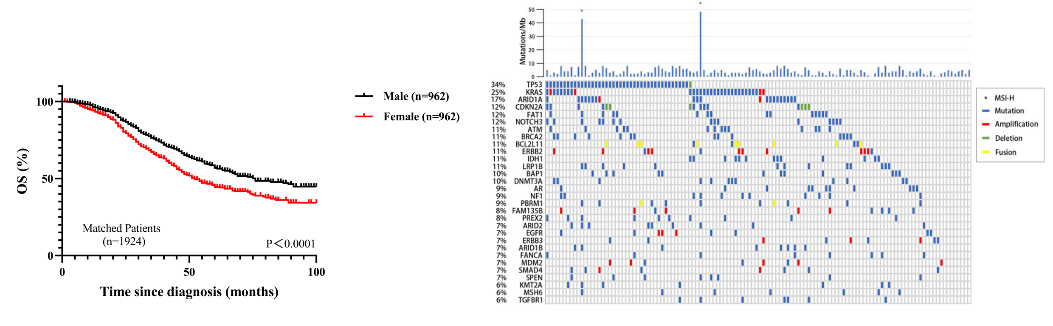
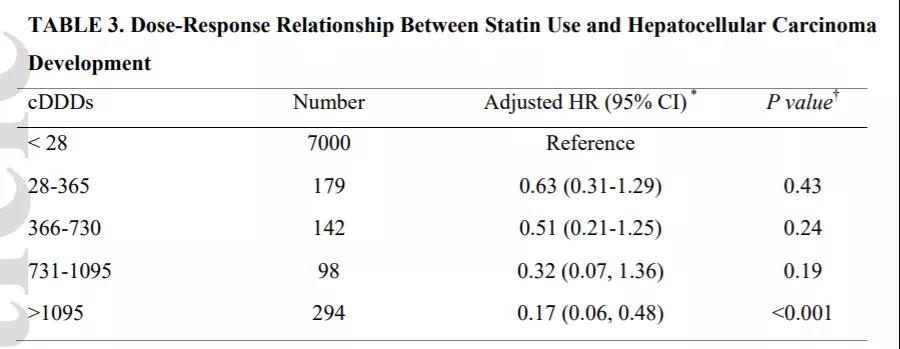
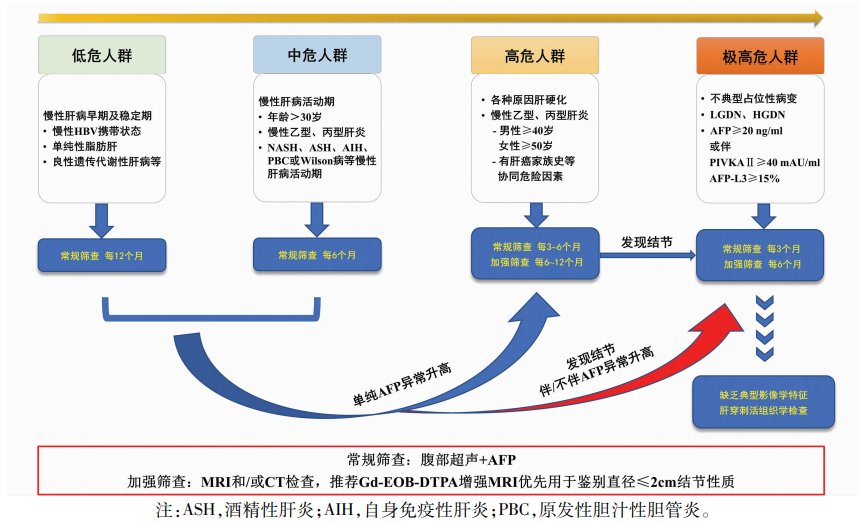
参考文献ZDI帝国网站管理系统
(在框内滑动手指即可浏览)ZDI帝国网站管理系统
ZDI帝国网站管理系统
[1] Valle JW,Kelley RK,Nervi B,et al.Biliary tract cancer[J].Lancet,2021,397(10272):428-444.ZDI帝国网站管理系统
[2] Nagino M,Ebata T,Yokoyama Y,et al.Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections[J].Ann Surg,2013,258(1):129-140.ZDI帝国网站管理系统
[3] Portnow LH,Kochkodan-Self JM,Maduram A,et al.Multimodality imaging review of HER2-positive breast cancer and response to neoadjuvant chemotherapy[J].Radiographics,2023,43(2):e220103.ZDI帝国网站管理系统
[4] Chalabi M,Fanchi LF,Dijkstra KK,et al.Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers[J].Nat Med,2020,26(4):566-576.ZDI帝国网站管理系统
[5] Marron TU,Fiel MI,Hamon P,et al.Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial[J].Lancet Gastroenterol Hepatol,2022,7(3):219-229.ZDI帝国网站管理系统
[6] Kuriyama N,Usui M,Gyoten K,et al.Neoadjuvant chemotherapy followed by curative-intent surgery for perihilar cholangiocarcinoma based on its anatomical resectability classification and lymph node status[J].BMC Cancer,2020,20(1):405.ZDI帝国网站管理系统
[7] Matsuyama R,Mori R,Ota Y,et al.Impact of gemcitabine plus S1 neoadjuvant chemotherapy on borderline resectable perihilar cholangiocarcinoma[J].Ann Surg Oncol,2022,29(4):2393-2405.ZDI帝国网站管理系统
[8] Shroff RT,Javle MM,Xiao L,et al.Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial[J].JAMA Oncol,2019,5(6):824-830.ZDI帝国网站管理系统
[9] Choi SH,Kang I,Lee SH,et al.Clinical feasibility of curative surgery after nab-paclitaxel plus gemcitabine-cisplatin chemotherapy in patients with locally advanced cholangiocarcinoma[J].Surgery,2023,173(2):280-288.ZDI帝国网站管理系统
[10] Maithel SK, Javle MM,Mahipal A, et al. NEO-GAP: A phase II single-arm prospective feasibility study of neoadjuvant gemcitabine/cisplatin/nab-paclitaxel for resectable high-risk intrahepatic cholangiocarcinoma[J].J Clin Oncol,2022,40(suppl16):4097.ZDI帝国网站管理系统
[11] Subbiah V,Lassen U,Elena Élez,et al.Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial[J].Lancet Oncol,2020,21(9):1234-1243.ZDI帝国网站管理系统
[12] Ohba A, Morizane C, Kawamoto Y,et al. Trastuzumab deruxtecan(T-DXd; T-DXd)/ target=_blank class=infotextkey>DS-8201)in patients(pts)with HER2-expressing unresectable or recurrent biliary tract cancer(BTC): An investigator-initiated multicenter phase 2 study(HERB trial)[J]. J Clin Oncol,2022,40(suppl 16):4006.ZDI帝国网站管理系统
[13] Tine BAV,Hubbard JM, Mita MM, et al. A phase 1 study of the novel immunotoxin MT-5111 in patients with HER2+ tumors: Interim results[J]. J Clin Oncol,2022,40(suppl 16):2583.ZDI帝国网站管理系统
[14] Ikeda S,Kudo R, Yamashita Y,et al. Primary results from JUPITER, a phase 2 basket trial of combination therapy with trastuzumab and Pertuzumab in patients with HER2-amplified solid tumors[J]. J Clin Oncol,2022,40(suppl 16):3131.ZDI帝国网站管理系统
[15] Harding JJ, Piha-Paul SA,Shah RH,et al. Targeting HER2 mutation-positive advanced biliary tract cancers with neratinib: Final results from the phase 2 SUMMIT basket trial[J]. J Clin Oncol,2022,40(suppl 16):4079.ZDI帝国网站管理系统
[16] Meric-Bernstam F,Hamilton EP,Beeram M,et al. Zanidatamab(ZW25)in HER2-expressing gastroesophageal adenocarcinoma(GEA): Results from a phase I study[J]. J Clin Oncol,2021,39(supp l3):164.ZDI帝国网站管理系统
[17] Meric-Bernstam F,Hainsworth J,Bose R,et al. MyPathway HER2 basket study: Pertuzumab(P)+ trastuzumab(H)treatment of a large,tissue-agnostic cohort of patients with HER2-positive advanced solid tumors[J]. J Clin Oncol,2021,39(suppl 15):3004.ZDI帝国网站管理系统
[18] Skoulidis F,Li BT,Dy GK,et al.Sotorasib for lung cancers with <i>KRAS</i> p.G12C mutation[J].KRAS,2021,384(25):2371-2381.ZDI帝国网站管理系统
[19] 谢智华,程庆保,姜小清.胆道恶性肿瘤免疫治疗进展[J].中国实用外科杂志,2021,41(9):1056-1061.ZDI帝国网站管理系统
[20] Chen X,Wu X,Wu H,et al.Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial[J].J Immunother Cancer,2020,8(2):e001240.ZDI帝国网站管理系统
[21] Oh DY,Lee KH,Lee DW,et al.Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study[J].Lancet Gastroenterol Hepatol,2022,7(6):522-532.ZDI帝国网站管理系统
[22] III HAB,Okusaka T, Vogel A, et al. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer[J]. J Clin Oncol,2022,40(suppl16):4070.ZDI帝国网站管理系统
[23] Vogel A,Chen L-T,He AR, et al. Regional subgroup analysis of the phase 3 TOPAZ-1 study of durvalumab(D)plus gemcitabine and cisplatin(GC)in advanced biliary tract cancer(BTC)[J]. J Clin Oncol,2022,40(suppl16):4075.ZDI帝国网站管理系统
[24] Li W,Wang Y,Yu Y, et al. Toripalimab combined with gemcitabine and S-1 in the first-line treatment of advanced biliary tract cancer[J]. J Clin Oncol,2022,40(suppl 16):4081.ZDI帝国网站管理系统
[25] Jung JH,Lee HJ,Lee HS,et al.Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma[J].World J Gastroenterol,2017,23(18):3301-3308.ZDI帝国网站管理系统
[26] Kobayashi S,Tomokuni A,Gotoh K,et al.A RETrospective analysis of the clinical effects of neoadjuvant combination therapy with full-dose gemcitabine and radiation therapy in patients with biliary tract cancer[J].Eur J Surg Oncol,2017,43(4):763-771.ZDI帝国网站管理系统
[27] Nakashima S,Kobayashi S,Nagano H,et al.BRCA/Fanconi anemia pathway implicates chemoresistance to gemcitabine in biliary tract cancer[J].Cancer Sci,2015,106(5):584-591.ZDI帝国网站管理系统
[28] Mansoori B,Mohammadi A,Doustvandi MA,et al.Photodynamic therapy for cancer: Role of natural products[J].Photodiagnosis Photodyn Ther,2019,26:395-404.ZDI帝国网站管理系统
[29] Cheon YK,Lee TY,Lee SM,et al.Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma[J].HPB (Oxford),2012,14(3):185-193.ZDI帝国网站管理系统
[30] Hong MJ,Cheon YK,Lee EJ,et al.Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma[J].Gut Liver,2014,8(3):318-323.ZDI帝国网站管理系统
[31] Stieber AC,Marino IR,Iwatsuki S,et al.Cholangiocarcinoma in sclerosing cholangitis. The role of liver transplantation[J].Int Surg,1989,74(1):1-3.ZDI帝国网站管理系统
[32] Meyer CG,Penn I,James L.Liver transplantation for cholangiocarcinoma: results in 207 patients[J].Transplantation,2000,69(8):1633-1637.ZDI帝国网站管理系统
[33] Robles R,Figueras J,Turrión VS,et al.Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma[J].Ann Surg,2004,239(2):265-271.ZDI帝国网站管理系统
[34] Alden ME,Mohiuddin M.The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer[J].Int J Radiat Oncol Biol Phys,1994,28(4):945-951.ZDI帝国网站管理系统
[35] De Vreede I,Steers JL,Burch PA,et al.Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma[J].Liver Transpl,2000,6(3):309-316.ZDI帝国网站管理系统
[36] Sudan D,DeRoover A,Chinnakotla S,et al.Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma[J].Am J Transplant,2002,2(8):774-779.ZDI帝国网站管理系统
[37] Darwish Murad S,Heimbach JK,Gores GJ,et al.Excellent quality of life after liver transplantation for patients with perihilar cholangiocarcinoma who have undergone neoadjuvant chemoradiation[J].Liver Transpl,2013,19(5):521-528.ZDI帝国网站管理系统
[38] Heimbach JK,Gores GJ,Haddock MG,et al.Liver transplantation for unresectable perihilar cholangiocarcinoma[J].Semin Liver Dis,2004,24(2):201-207.ZDI帝国网站管理系统
[39] Rea DJ,Heimbach JK,Rosen CB,et al.Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma[J].Ann Surg,2005,242(3):451-461.ZDI帝国网站管理系统
[40] Azad AI,Rosen CB,Taner T,et al.Selected patients with unresectable perihilar cholangiocarcinoma (pCCA) derive long-term benefit from liver transplantation[J].Cancers (Basel),2020,12(11):3157.ZDI帝国网站管理系统
[41] Murad SD,Ray Kim W,Harnois DM,et al.Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers[J].Gastroenterology,2012,143(1):88-98.ZDI帝国网站管理系统
[42] Zaborowski A,Heneghan HM,Fiore B,et al.Neoadjuvant chemoradiotherapy and liver transplantation for unresectable hilar cholangiocarcinoma: The irish experience of the mayo protocol[J].Transplantation,2020,104(10):2097-2104.ZDI帝国网站管理系统
[43] Ebata T,Mizuno T,Yokoyama Y,et al.Surgical resection for Bismuth type IV perihilar cholangiocarcinoma[J].Br J Surg,2018,105(7):829-838.ZDI帝国网站管理系统
[44] Breuer E,Mueller M,Doyle MB,et al.Liver transplantation as a new standard of care in patients with perihilar cholangiocarcinoma? results from an international benchmark study[J].Ann Surg,2022,276(5):846-853.ZDI帝国网站管理系统
[45] Wong M,Kim J,George B,et al.Downstaging locally advanced cholangiocarcinoma pre-liver transplantation: A prospective pilot study[J].J Surg Res,2019,242:23-30.ZDI帝国网站管理系统
[46] Twohig P,Peeraphatdit TB,Mukherjee S.Current status of liver transplantation for cholangiocarcinoma[J].World J Gastrointest Surg,2022,14(1):1-11.ZDI帝国网站管理系统
[47] Lunsford KE,Javle M,Heyne K,et al.Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series[J].Lancet Gastroenterol Hepatol,2018,3(5):337-348.ZDI帝国网站管理系统
[48] Ito T,Kuriyama N,Kozuka Y,et al.High tumor budding is a strong predictor of poor prognosis in the resected perihilar cholangiocarcinoma patients regardless of neoadjuvant therapy, showing survival similar to those without resection[J].BMC Cancer,2020,20(1):209.ZDI帝国网站管理系统
[49] Yadav S,Xie H,Bin-Riaz I,et al.Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis[J].Eur J Surg Oncol,2019,45(8):1432-1438.ZDI帝国网站管理系统
[50] Mac Manus MP,Hicks RJ,Matthews JP,et al.Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer cORRelates with patterns of failure[J].Lung Cancer,2005,49(1):95-108.ZDI帝国网站管理系统
[51] Weber WA,Petersen V,Schmidt B,et al.Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use[J].J Clin Oncol,2003,21(14):2651-2657.ZDI帝国网站管理系统